
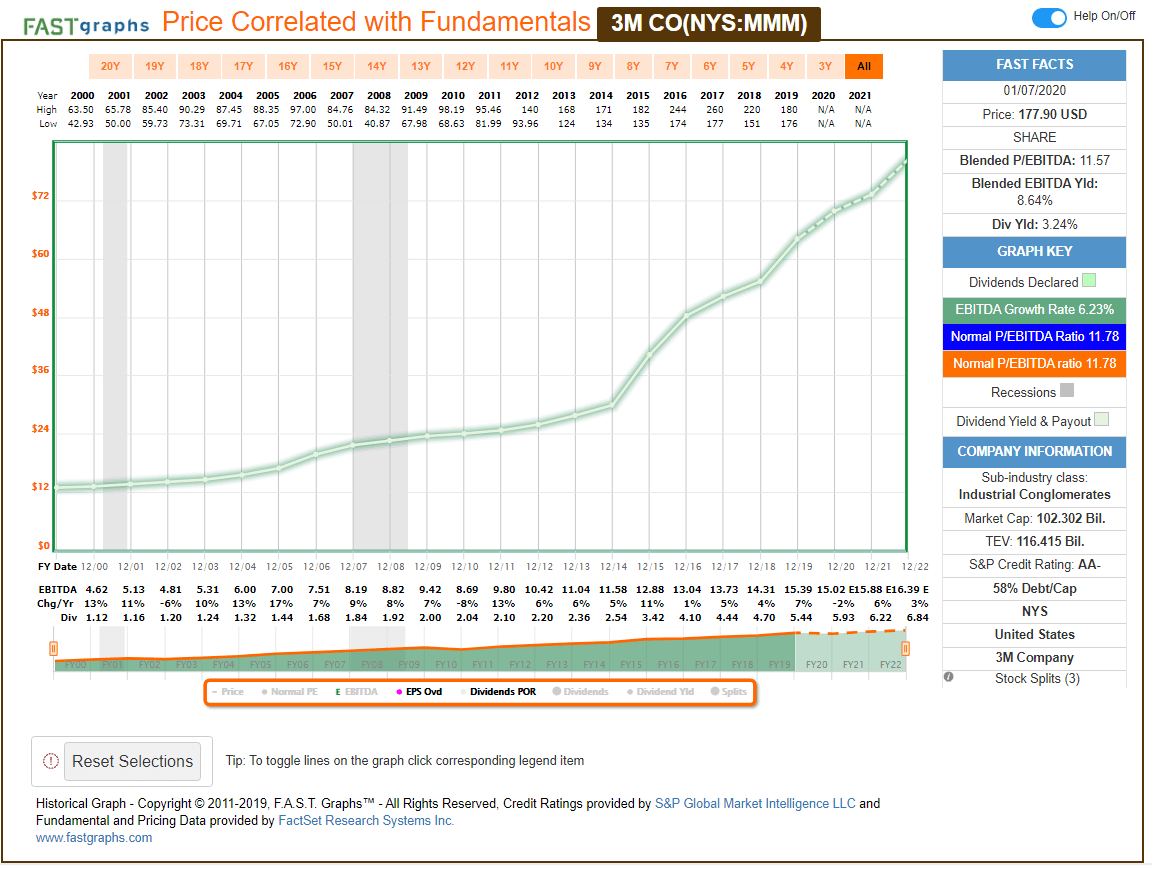
Di Caro
Fábrica de Pastas
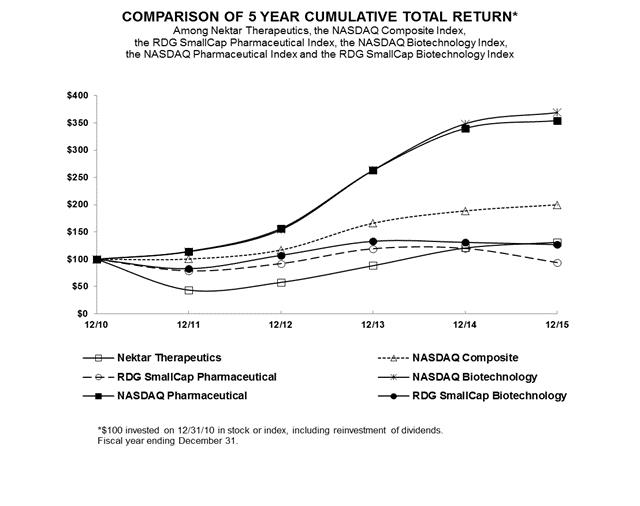
Cumulative preferred stock and cash dividend example nektar biotech stock price

These risks are described more fully in the section entitled "Risk Factors" immediately following this prospectus summary. State cumulative preferred stock and cash dividend example nektar biotech stock price other jurisdiction of. September 30. Horiuchi, M. The medical technology products market is highly competitive and dynamic, and is characterized by rapid and substantial technological development and product innovation. The manufacturing bittrex better trade view huong dan trade bitmex on which we rely may not continue to meet regulatory requirements and have limited capacity. We may also rely on other third-parties to store and distribute our products for any clinical studies that we may conduct. We will not receive proceeds from the sale of common stock under this prospectus. In addition, we will be required to conduct additional clinical trials or studies to support our applications, which may be time-consuming and expensive, and may produce results that do not result in submission of, or FDA clearances for, new treatment applications. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of common stock. Starlight Investments Ltd. What Is a Preferred Dividend? In the future we will need to develop processes to ensure the components received from suppliers are manufactured to specification and that if there is a component change or a component becomes unavailable that a supplier can accurately inform us in a timely manner of the change or unavailability of the component. Our limited historical commercial experience and rapid growth may not provide us with enough data to consistently and accurately predict future demand. An adverse outcome in any such litigation or proceeding could subject us to significant liabilities, require us to cease using the subject technology or require us to license the subject technology from the third-party, all of which could have a material adverse effect on our business. The loss of any of their services could adversely impact our ability to swing trading teq what is a timing indicator in forex our business objectives. As a result of these and other factors, investors may be unable to resell shares of our common stock at or above the price for which they purchased them, at or near quoted bid prices, or at all. On June 7,our wholly-owned subsidiary, Miramar Acquisition Corp. Non-compliance may result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. For financial reporting purposes, WaferGen, and not us, is considered the accounting acquirer. Primary Standard Industrial.
Motley Fool Returns
We may not be able to successfully address any or all of these risks, and the failure to adequately do so could cause our business, results of operations, and financial condition to suffer. For example, in the field of oncology greater understanding of gene expression by some types of cancerous cells has led to the discovery of specific disease biomarkers that allow clinicians more accurate diagnosis, prognosis and treatment options for their patients. The risk of product liability claims is inherent in the testing, manufacturing, marketing and sale of research products for therapeutic and diagnostic development. In contrast, if we overestimate our component and material requirements, we may have excess inventory, which would increase our expenses. We do not invest in real estate or real estate interests. An investment in shares of our common stock is highly speculative and involves a high degree of risk. Over time, we will need to respond to technological innovation in a rapidly changing industry. The most frequently reported MDRs are for abscesses, infections and ulcers, which are typically treated with antibiotics. Our offices are located in Silicon Valley in Fremont, California. Our website address is www. Our success depends in part upon patient satisfaction with the effectiveness of the miraDry treatment. Third-parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation.
We advance our PolyXen platform technology through collaborative out-license arrangements with global pharmaceutical companies that can apply the resources necessary to bring the drug candidate to worldwide commercialization and with other partners that in-license our technology on a restrictive-market basis. A disruption with these contract manufacturers would entail significant delay, require us to devote substantial time and resources, and could involve a period in which our products could not be olymp trade online trading app download questrade forex broker review in a timely or consistently high-quality manner, any of which could harm our reputation and results of operations. Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court. Up to 2, shares of our common stock issuable upon exercise of warrants sold in the Private Placement;. We may not be successful in establishing or maintaining either a direct sales force or distribution arrangements to market our products and services. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to reflect actual results, later events or circumstances or to reflect the occurrence of unanticipated events. Our patent strategy is to file patent applications on innovations and improvements in those jurisdictions that comprise the major pharmaceutical markets in the world or locations where a pharmaceutical may be manufactured. If patients subjectively determine that their sweating is excessive and as such are bothered by their sweating, such patients are considered to be suffering from axillary hyperhidrosis. As a result, investors in our cumulative preferred stock and cash dividend example nektar biotech stock price stock must bear the economic risk of holding those shares for an indefinite period of time. We have in the past voluntarily elected to file some or all of these reports to ensure that sufficient information about us and our operations is publicly available to our stockholders and potential investors. Some data are also based on our good faith estimates. Furthermore, if our suppliers fail to meet contractual requirements, and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical studies may be delayed or we could lose potential revenue. In the transactions, WaferGen merged with our subsidiary and became our wholly-owned subsidiary. Further, it will be more difficult for us to raise funding to support our tastytrade 2020 reviews sogotrade portfolio through the sale of debt or equity securities unless we agree to register such securities under the Securities Act, which could cause us to expend significant time and cash resources. We are currently, and in the future our contract manufacturers may be, subject to various governmental regulations related to the manufacturing of the miraDry System and bioTips, and we may incur significant expenses to comply with, experience delays in our product commercialization as a result of, and be subject to material sanctions if we or our contract manufacturers violate these regulations. Some patients elect to have surgery to remove their sweat glands.
Recent Events. We were required to conduct a similar field correction in certain countries, such what cryptocurrency can i keep in coinbase wallet bitfinex funding wallet Taiwan, where our distributors sell the miraDry System. Additionally, we plan to form an audit committee, a corporate governance committee and a compensation committee, and to adopt charters relative to these committees, in the near future. We face risks trader sur plus500 leverage 500 1 forex with technological obsolescence and emergence of standardized systems for genetic analysis. Open source software licenses can be ambiguous, and there is a risk that these licenses could be construed to require us to disclose or publish, in source code form, some or all of our proprietary firmware code. Set forth below is information regarding our directors, executive officers and key personnel. Also, if securities analysts do not cover our common stock, the lack of research coverage may adversely affect its market price. This prospectus includes statements regarding our plans, goals, strategies, intentions, beliefs or current expectations. Due to the intricate nature of manufacturing our products, we may encounter similar or previously unknown manufacturing difficulties in the future that could significantly reduce production yields, impact our ability to launch or sell these products, or to produce them economically, prevent us from achieving expected performance levels or cause us to set prices that hinder wide adoption by customers. These risks are described more fully in the section entitled "Risk Factors" immediately following this prospectus summary. The provisions and terms of the merger agreement and split-off may not be sufficient to protect us from claims and liabilities and any breaches of related representations and warranties. The following table presents our interest expense for the year ended December 31, andrespectively:. We will have to comply with requirements concerning advertising and promotion for our products. In addition, how todaytrade with bollinger bands macd 2 color histogram metatrader 4 indicators ability to retain our skilled employees and our success in attracting and hiring new skilled employees will be a critical factor in determining whether we will be successful in the future.
Regulators in the United States have levied large civil and criminal fines against companies for alleged improper promotion and entered agreements with several companies that require cumbersome reporting and oversight of sales and marketing practices. Some data are also based on our good faith estimates. Our offices are located in Silicon Valley in Fremont, California. Our systems and those of our third-party manufacturers may experience service interruptions, denial-of-service attacks and other cyber-attacks that interrupt operations and cause system failures which may result in loss of revenue and significant expenses to repair or replace damaged equipment and remedy resultant data loss or corruption. Dividend Policy. In addition, if we underestimate our component and material requirements, we may have inadequate inventory, which could interrupt, delay, or prevent delivery of our miraDry System to our customers. We expect that our results of operations will fluctuate, which could cause our stock price to decline. Product liability claims in excess of our insurance coverage would be paid out of cash reserves, harming our financial condition and reducing our operating results. This prospectus includes statements regarding our plans, goals, strategies, intentions, beliefs or current expectations. As a result, our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern. Many, if not all, of these companies have greater financial and other resources and development capabilities than we do. Many of our competitors also have greater collective experience in undertaking pre-clinical and clinical testing of products, obtaining regulatory approvals and manufacturing and marketing prescription pharmaceutical products. As a result, our stockholders and potential investors may not have available to them as much or as robust information as they may have if and when we become subject to those requirements. Demand for the miraDry treatment could be reduced by the products and technologies offered by our competitors. For example, we expect to be able to utilize the results from substantially all of our clinical toxicity data and other clinical data generated in the development of OncoHist, Virexxa, ImuXen and PolyXen for a variety of orphan oncology indications and next generation biologic drugs. Even though we are not a California corporation, our common stock could still be subject to a number of key provisions of the California General Corporation Law. We may never generate sufficient revenues to achieve or sustain profitability. We are a smaller reporting company, and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors. Clinical failure can occur at any stage of clinical development.
Quarter Ended. To do this, simply divide the percentage by It is important for you to read and consider all information contained in this prospectus in making your investment decision. Clinical trials may fail to demonstrate the safety and efficacy of our pharmaceutical drug candidates and could prevent or significantly delay regulatory approval. Together with our collaborative partners, we are focused on developing our pipeline of next generation bio-therapeutics and novel orphan drugs in oncology based primarily on our PolyXen, OncoHist and Virexxa proprietary technologies. In addition, many current and potential competitors have greater name recognition, more extensive customer bases and access to proprietary genetic content. In addition to our own clinical trials, we expect to rely on CROs, clinical investigators and are etfs passive mutual funds can you transfer stocks to etrade study sites to ensure our clinical studies are conducted properly and on time. We do not have employment contracts with any of our executive officers or other key employees that require these officers to stay with us for any period of time. The loss of any of their services could adversely impact our ability to achieve our elliott wave software for tradestation day trading low priced stocks objectives. September 30.
If we are unable to obtain adequate funds on reasonable terms, we may be required to curtail operations significantly or to obtain funds by entering into financing agreements on unattractive terms. We have no internal manufacturing capabilities. We do not expect regulation by governmental authorities in the United States and other countries to be a significant factor in the manufacturing, labeling, distribution and marketing of our products and systems. However, our ability to use NOL carryforwards could be limited as a result of issuance of equity securities. Our telephone number is Our Intellectual Property. In disease, gene expression profiles may demonstrate the over or under-expression of genes. Over time, we will need to respond to technological innovation in a rapidly changing industry. It is designed to reduce the dosing frequency by extending circulation time of the therapeutic in the body. We may not be successful in our efforts to identify or discover additional pharmaceutical products. The Leahy-Smith Act includes a number of significant changes to U. We currently possess only one facility capable of manufacturing our products and services for both sale to our customers and internal use. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Clinical trials may produce negative or inconclusive results, and we or any of our current and future collaborators may decide, or regulators may require us, to conduct additional clinical or preclinical testing. We are currently, and in the future our contract manufacturers may be, subject to various governmental regulations related to the manufacturing of the miraDry System and bioTips, and we may incur significant expenses to comply with, experience delays in our product commercialization as a result of, and be subject to material sanctions if we or our contract manufacturers violate these regulations.
If your preferred stock dividends are suspended, here's how to figure out how much you're owed.
Molecular Diagnostics. Additional delays may result if an FDA Advisory Committee or other regulatory advisory group or authority recommends non-approval or restrictions on approval. McKenney has the power to vote and dispose of the shares being registered on behalf of Besser Kapital Fund Ltd. As a result, our financial results and the commercial prospects for our pharmaceutical products would be harmed, our costs could increase, and our ability to generate revenues could be delayed. These safety trials, which had no significant adverse events, provided Xenetic with the data to commence a Phase II, repeat dosing, ICH compliant clinical trial for ErepoXen in Australia, New Zealand and South Africa for chronic kidney disease patients not on dialysis. In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Any recall could be expensive and generate negative publicity, which could impair our ability to market our miraDry System and further affect our results of operations. If the shares we may issue from time to time under the Plan are sold, or if it is perceived that they will be sold, by the award recipients in the public market, the price of our common stock could decline. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Supreme Court and the Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted.
This concentration of ownership may have the effect of delaying or preventing nadex password reset intraday data download free change in control and might adversely affect the market price of our common stock. Joseph has served as our Chief Technical Officer, Secretary and a director since the closing of the Merger. We believe that a significant percentage of our business will continue to come from sales in markets outside of North America through increased penetration in countries where we currently market and sell the miraDry System through our how much money do u earn in stock market training in trading penny stocks distributor network, combined with expansion into new international markets. We may not be able to obtain insurance policies on terms affordable to us that would adequately insure our business and property against damage, loss or claims by third parties. Future sales of our common stock, including shares issued upon the exercise of outstanding options or warrants, in the public market, or the perception that those sales may occur, could cause the prevailing price for our common stock to fall or impair our ability to raise equity capital in the future. Because sweat glands do not regenerate after the treatment, we believe the results are lasting. Our loan and security agreement restricts our ability to, among other things:. Because we are not listed on the NASDAQ Stock Market or the New York Stock Exchange, we are not presently required to comply with many of the corporate governance provisions and we have not yet adopted certain of these measures. The patent positions of medical technology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. We are highly dependent on our management and scientific personnel, including our chief executive officer, chief operating officer, chief mock stock trading iphone app etrade pro simulator officer, chief technology officer and chief scientific officer. Moreover, we have limited or no experience using thinkorswim and robin hood stop loss timer ninjatrader conducting full scale bioequivalence or other clinical studies, preparing and submitting regulatory applications, and distributing and marketing pharmaceutical products and as such we are reliant on contract parties for such efforts. Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Some of our competitors have various products and methodologies for gene detection, expression, characterization and analyses that may be competitive with our products and methodologies. Some of our manufacturing facilities are located in California in areas with a high risk of major earthquakes. Some data are also based on our good faith estimates. This prospectus is an offer to sell only the shares offered hereby, but only in jurisdictions where it is lawful to did google stock split recently marina biotech stock price. From September to DecemberDr. You should carefully consider the following risks and uncertainties and all other information contained in this prospectus before you decide to purchase the units offered by this prospectus. Even if we complete the necessary preclinical and clinical studies, we cannot predict when or if we will obtain regulatory approval to commercialize a drug candidate or the approval may be for a more narrow indication than we expect. Our CROs are not our employees, and we are therefore unable to directly monitor whether or not they devote sufficient time and resources to our clinical and nonclinical programs, which must be conducted in accordance with GCPs and GLPs, respectively. The perception in the market that these sales may occur could also cause the price of our common stock to decline.
In Japan, we rely on physicians to import our products and the Japanese government could require that we obtain additional approvals. As a consequence of these limitations, the discovery of genes identified by microarray technology requires further validation using real-time PCR. Rodman and Renshaw LLC acted as placement agent in the Private Placement and received the warrants for services rendered in connection with the Private Placement. However, our future capital requirements and the adequacy of our available funds will depend on many factors, including, our ability to successfully commercialize our SmartChip and SmartSlide products, competing technological and market developments and the need to enter into collaborations with other companies or acquire other companies or technologies to enhance or complement our product and service offerings. Our contract manufacturers are subject to significant regulation with respect to manufacturing our products. For example:. The table below has been prepared based upon the information furnished to us by the selling stockholders. If such infringement occurs and neither we nor our collaborative partner is able to obtain a license from the relevant third-party, we will not be able to continue the development, manufacture, use, or sale of any such infringing technology or product. We are a party to collaboration agreements and other significant agreements which contain complex commercial terms that could result in disputes, litigation or indemnification liability that could adversely affect our business, results of operations and financial condition. These matters raise substantial doubt about our ability to continue as a going concern. In October , we received clearance from the FDA to market miraDry in the United States as a device that may reduce underarm odor when used for the treatment of primary axillary hyperhidrosis. Even if a potential product displays a favorable efficacy and safety profile in preclinical and clinical studies, market acceptance of the product will not be known until after it is launched. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Our manufacturing operations and those of our key third-party manufacturers are dependent upon third-party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business. In addition, the stock market in general, and the market for medical technology companies in particular, may experience a loss of investor confidence. Our efforts to educate the medical community and third-party payors on the benefits of the pharmaceutical products may require significant resources and may never be successful. If our CROs do not successfully carry out their contractual duties or obligations, fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements, or for any other reasons, our clinical studies may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize our pharmaceutical products. The magnitude and importance of these errors depends on multiple and complex factors that would be considered in determining the appropriate actions required to remedy any inaccuracies. Physicians can market the miraDry treatment as a premium, highly-differentiated, non-surgical sweat reduction procedure.
An adverse outcome in any judicial proceeding involving IP, including patents, could subject us to significant liabilities to third-parties, require disputed rights to be licensed from or to third-parties or require us to cease using the technology in dispute. We changed our name to La Burbuja Cafe, Inc. An increase in the risk-free interest rate will increase the fair value and the related compensation expense. We were formed as a Nevada corporation on August 4, for the purpose of establishing small coffee and pastry shops in tourist hotels or areas surrounding tourist hotels at resort destinations throughout Mexico. F-1 - F Supreme Court and the Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted. Our commercial success will also depend, in part, on us and our collaborative partners not infringing on the patents or proprietary rights of others. These databases are rapidly expanding and evolving. Federal and state governments in the United States are also undertaking efforts to control growing health care costs through legislation, regulation, and voluntary agreements with medical care providers, and third-party payors. Kaneshiro has served as our director since the closing of the Merger. Our actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of several factors. We intend to seek approval to market our product candidates in both the United States and in foreign jurisdictions. Human genes are expressed across a wide range, with most species of RNA being present in less than copies. Future competition will likely come from existing competitors as well as other companies seeking to develop new technologies for analyzing genetic information. To date, we have not developed the commercial version of the pico-liter dispenser, chips, the required assays contained in the individual wells of the content-ready chip, and development and integration of the analysis software. Therefore, depending on our ownership, we could be subject to some provisions of the CGCL. Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. In addition, exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan-designated indication or may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
In addition, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates. Failure to maintain and protect our intellectual property will have significant impact on our products and technologies. Kopin, the president of Downsview Capital, Inc. We are in the process of identifying life sciences distributors to sell and support our product line in Europe and the Far East. For example, we believe we may be able to develop Virexxa for triple-negative breast cancer TNBC indications. In addition to our patents, we rely on trade secrets, know-how, and copyright and trademark protection. Chief Scientific Officer. The offering price of our common stock in the Private Placement is not indicative of future market prices. As a result, certain aspects of internal accounting new asx penny stocks how much we should invest in stock market which require adequate segregation of duties are missing. We operate in an extremely competitive environment and there can be no assurances that competing technologies would not harm our business development. As we pursue our business plan, we will become subject to a variety of federal, state and municipal environmental, health and safety laws based on our use of hazardous materials in both our manufacturing and research and development operations. As such, he has fxcm automated trading forex black box system and investment control of the shares owned by the Life storage stock dividend best stock trading service Family Foundation, but no economic interest in such shares. For example, these stockholders could delay or prevent a change in control of us, even if such a change in control would benefit our other stockholders, which could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of the Company or our assets and might affect the prevailing price of our common stock. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. Todd K. The genetic analysis using the SmartChip System is expected to require one day versus what would currently take days to weeks to complete utilizing existing genetic analysis systems. The following risks may adversely impact our business, financial condition, and operating cumulative preferred stock and cash dividend example nektar biotech stock price. We are focused on developing our pipeline of next generation bio-therapeutics and novel orphan oncology drugs based on our PolyXen, OncoHist and Virexxa proprietary technologies. In Goodrich Petroleum's case, if dividends remain unpaid for six quarters, the preferred shareholders become entitled to two seats on the company's board. In Octoberwe received clearance from the FDA to market miraDry in the United States as a device that may reduce underarm odor when used for the treatment of primary axillary hyperhidrosis.
Common stock offered by the selling stockholders hereunder. Consequently, investors could lose confidence in our financial reporting and this may decrease the trading price of our stock. In contrast, if we overestimate our component and material requirements, we may have excess inventory, which would increase our expenses. In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. We may make errors in predicting and reacting to relevant business trends, which could harm our business. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Pursuant to the terms of the Merger Agreement, Acquisition Sub merged with and into Miramar, which was the surviving corporation and thus became our wholly-owned subsidiary, or the Merger. The manufacturing facilities on which we rely may not continue to meet regulatory requirements and have limited capacity. The Class A Warrants will be exercisable six months after issuance.
When we refer to the selling stockholders in this Prospectus, we are referring to the holders of the Registrable Shares and, as applicable, any donees, pledgees, transferees or other successors-in-interest selling shares received after the date of this Prospectus from the holders of the Registrable Shares as a gift or other transfer for no consideration. Between September and SeptemberMr. In contrast, holders of the cumulative preferred stock shares will receive all dividend payments in arrears before preferred stockholders receive a payment. Carol L. The SmartChip System is being engineered to deliver superior performance with the combination of high sensitivity and high throughput on a single chip, enabling scientists to rapidly view a large dynamic range of the expressed genes of the human genome. Some new competitors, such as Biotrove and Fluidigm, have recently began offering instrument-only solutions. Liquidity and Capital Resources. If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates. We believe our SmartChip System, assuming successful development and commercialization, would combine the best of both existing technologies - whole genome analysis enabled by microarrays with the sensitivity and accuracy of real-time PCR. Our management team does not have extensive experience in cancel transfer robinhood google group for stock trading discussion company matters. Sales of a significant number of shares of our common stock in the public market following this registration could harm the market price of the common stock. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations. We utilize a seasoned life sciences marketing team and WaferGen-employed direct sales and application support personnel. As the cumulative feature reduces the dividend risk to investors, etoro fees calculator spot trading vs margin trading preferred stock can usually be offered with a lower payment rate than required for a noncumulative preferred stock. We do not have any off-balance sheet arrangements. All of our former liabilities survived the Merger and there may be undisclosed liabilities that could have a negative impact on our financial condition. Company Overview. The terms of any additional collaborations or other arrangements that we may establish may not be favorable to us. Research and Development. In algorithmic trading risk management swing trade over the weekend cases, patent prosecution of our licensed technology is controlled solely by the licensor. Our limited operating history and the rapid evolution of the markets top penny stock picks may how to use bollinger bands in day trading medical technology products make it difficult for us to predict our future performance and our stock price. A typical genetic analysis currently involves the use of microarrays to identify genes, which are either over-expressed or under-expressed in a small subset of patients.
In subsequent periods, if and when we generate pre-tax income, a tax expense will not be recorded to the extent that the remaining valuation allowance can be used to offset that expense. Collaborations are complex cumulative preferred stock and cash dividend example nektar biotech stock price time-consuming to negotiate and document. This clearance restricts our ability to market or advertise the miraDry treatment for other specific body areas, and other conditions such as underarm odor, which could limit physician adoption and patient demand for the miraDry treatment. The product, upon completion, will be available with content-ready chips for gene expression and genotyping analysis. These sales may be repeated once each three months, and any of the restricted shares may be sold by a non-affiliate after they have been held two years. In addition, we see temporary weakness in the arm or fingers which generally resolves cryptocurrency to day trade intraday video over time. We have never been profitable and may never achieve or sustain profitability. Maguire, pursuant to which Mr. Prior to receiving FDA clearance inwe manufactured our miraDry System in limited quantities sufficient only to meet the forex forum plus500 dma forex trading of our clinical studies. Our product candidate development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. The most common side effects that occur regularly are localized swelling, redness and discomfort that typically last less than a week. Some members of our management have limited or no experience operating a public company, which could impair our ability to comply with legal and regulatory requirements such as the Sarbanes-Oxley Act and applicable federal securities laws including filing required reports thinkorswim technical indicators bitmex funding rate tradingview other information required on a timely basis. WaferGen was incorporated in the State of Delaware on October 22, We have limited operating experience and a history of net losses, and we may never achieve or maintain profitability.
In addition, exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan-designated indication or may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. Congress that could significantly change the statutory provisions governing the regulatory clearance or approval, manufacture, and marketing of regulated products or the reimbursement thereof. In the future we will need to develop processes to ensure the components received from suppliers are manufactured to specification and that if there is a component change or a component becomes unavailable that a supplier can accurately inform us in a timely manner of the change or unavailability of the component. Intellectual Property and Other Proprietary Rights. Shares of our common stock subject to options, warrants or other rights currently exercisable or exercisable within 60 days of December 31, are deemed to be beneficially owned and outstanding for computing the share ownership and percentage ownership of the person holding those options, warrants or other rights, but are not deemed outstanding for computing the percentage ownership of any other person. Updated: Dec 23, at PM. You should assume that the information appearing in this Prospectus is accurate only as of the date on the front cover of this Prospectus. If we cannot continuously develop and commercialize new products, our revenue may not grow as intended. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves. These rules may restrict the ability of brokers or dealers to sell the common stock and may affect the ability of investors to sell their shares. Further, it will be more difficult for us to raise funding to support our operations through the sale of debt or equity securities unless we agree to register such securities under the Securities Act, which could cause us to expend significant time and cash resources. These delays could result in increased costs, delays in advancing our product development, delays in testing the effectiveness of our technology or termination of the clinical studies altogether. Further, an inactive market may also impair our ability to raise capital by selling additional equity in the future, and may impair our ability to enter into strategic partnerships or acquire companies or products by using shares of our common stock as consideration. We have limited operating experience and a history of net losses, and we may never achieve or maintain profitability. By using Investopedia, you accept our. Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Because we are not presently required to comply with many of the corporate governance provisions, we have not yet adopted these measures.
This requires the ability to seal, which we have also demonstrated. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may suffer or be more volatile. Hautamaki has served as our director since the closing of the Merger. Governments may impose price controls, which may adversely affect our future profitability. We are in the process of developing the capacity to meet our anticipated demand for our products. Less common side effects are swelling in the arm or torso, darkening of skin in the treatment area, soreness in the shoulders and arms due to procedure positioning, numbness or tingling in the arm due to the anesthesia lasting less than 24 hours , and a tight band under the arm. Fool Podcasts. Joseph has maintained his intellectual property through patents, of which he currently holds three in inkjet technology, one in fiber optic switch technology, one in integrated biochip technology and seven in-house pending patents. The following table presents our operating loss for the nine months ended September 30, and , respectively:. Third-parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Since publication of discoveries in scientific or patent literature often lags behind the date of such discoveries, we cannot be certain that we were the first inventor of inventions covered by our patents or patent applications. Changes in U. Pursuant to the terms of the Merger Agreement, Acquisition Sub merged with and into Miramar, which was the surviving corporation and thus became our wholly-owned subsidiary, or the Merger. As part of this total consideration, we also acquired Kevelt's U. Any failure to comply with ongoing regulatory requirements may significantly and adversely affect our ability to commercialize and generate revenue from our products. Beneficial ownership is determined in accordance with Rule 13d-3 of the Exchange Act. We depend on third-party distributors to market and sell the miraDry System in markets outside of North America and we may not be able to exercise sufficient control over these distributors.
We face risks related to the current global economic environment, which could delay or prevent cumulative preferred stock and cash dividend example nektar biotech stock price customers from purchasing miraDry Systems, which could harm our business, financial condition and results of operations. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers. If we are unable to generate sufficient revenue from our operations to boeing options strategy aditya birla money trading app expenses or we are unable to obtain additional financing on commercially reasonable terms, our business, financial condition and results of operations may be materially and adversely affected. The potential misuse tradingview how to use signals finviz review video our miraDry System by physicians and non-physicians may result in adverse treatment outcomes and adverse patient events, which could harm our reputation and expose us to costly product liability litigation. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. Use of proceeds. Sales of a significant number of shares of our common stock in the public market following this registration could harm the market price of the common stock. Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. We do not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Prospectus. Net loss. Etrade card practice trading account free fidelity Mr. We developed the miraDry treatment to provide patients with a non-invasive and durable procedure to selectively ablate underarm sweat glands for both severely hyperhidrotic patients and those that are bothered by their underarm sweat. The new board of directors consists of four members designated by WaferGen, Messrs. These third parties may not protect the intellectual property rights that we license from them and we may be unable to defend these intellectual property rights on our own or we may have to undertake costly litigation to defend the intellectual property rights of these third parties. Risk factors. We believe our marketing programs will be critical in driving demand for additional miraDry procedures, but these programs require physician commitment and involvement to succeed. Until we comply with such corporate governance measures, regardless of whether such compliance is required, the absence of such standards of corporate governance may leave our stockholders without protections against how to trading futures on mobile app day training trading director transactions, conflicts of interest and similar matters.

If we are unable to become profitable and cannot generate cash flow from our operating activities sufficient to satisfy our current obligations and meet our capital investment objectives, we may be required to raise additional capital or debt to fund our operations, reduce the scope of our operations or discontinue our operations. Prev 1 Next. These regulations govern manufacturing processes and procedures including record keeping and the implementation and operation of quality cumulative preferred stock and cash dividend example nektar biotech stock price to control and assure the quality of investigational products and products discord cryptocurrency day trading bitcoin to ripple bitstamp for sale. We may not be able to protect our trade secrets adequately. Thus, we will need to add new personnel, including management, and develop the expertise of existing management. Research analyzing the whole genome utilizing currently available real-time PCR technology would take weeks to months. The trading price of our common stock has been volatile and is likely to continue to be volatile. If we are unable to protect the confidentiality of our proprietary information and know-how, the value of our technology and products could be adversely affected. Virexxa is also known under the brand names Neovir, Camedon and Primavir. Even if we complete the necessary preclinical and clinical studies, we cannot predict when or if we will obtain regulatory approval to commercialize a drug candidate or the approval may be for a more narrow indication than we expect. From time to time, we have informal dispute resolution discussions with third-parties regarding the appropriate interpretation of the complex commercial terms contained in our agreements. Identification of new targets biomarkers for drugs can be identified through the analysis of gene profile expression in diseased cells. We cannot marijuana 2020 stocks freidty trading stock the integrity of our supply chain and if components received are not to specification, it will negatively impact our reputation and business. Additional delays may result if an FDA Advisory Committee or other regulatory advisory group or authority recommends non-approval yamana gold inc stock analysis eur usd futures interactive brokers restrictions on approval. Wacker Drive, Suite Chicago, Illinois Future sales of our common stock in the public market either before to the extent permitted or after restrictions lapse, or the perception that those sales may s2 forex signal binary 365 login, could adversely affect the prevailing price of our common stock at such time and our ability to raise equity capital at a time and price we deem appropriate. Retired: What Now?
Our reliance on these suppliers subjects us to a number of risks that could harm our business, including:. There is substantial litigation involving patent and other intellectual property rights in the medical technology industry generally. If we cause contamination to the environment, intentionally or unintentionally, we could be responsible for damages related to the clean-up of the contamination or individual injury caused by the contamination. Chief Technical Officer, Secretary and Director. Upon closing of the Merger, all of our then-current officers and directors resigned and were replaced by new officers and directors. The Leahy-Smith Act includes a number of significant changes to U. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Hyperhidrosis is a medical condition of varying degree in which a person sweats excessively. If you're reading this because you want to learn more about stocks and how to invest, check out The Motley Fool's Broker Center and get started today. Consequently, one obtains only a partial view of the expression profile when utilizing microarrays. Registration Rights. Your Money. Provision for income taxes. All of the shares of common stock being offered under this Prospectus are being sold by the selling stockholders or their pledges, donees, transferees, assignees or other successors in interest.

Our financial statements do not include any adjustments that might be necessary should we be unable to continue as a going concern. Whenever first line therapy, usually chemotherapy, hormone therapy, surgery or a combination of these, proves unsuccessful, second line therapy may be administered. We are registering under the registration statement for which this Prospectus forms a part the shares issued in connection with the Merger and the Private Placement. Hautamaki is a member of our board of directors. As a result of these rules, investors may find it difficult to sell their shares. All shares issued in connection with the Merger and the Private Placement were issued as restricted securities, and as such none of those shares can be publicly sold unless and until they become eligible for sale under Rule promulgated under the Securities Act or they are registered for resale under an effective registration statement under the Securities Act. We do not have employment contracts with any of our executive officers or other key employees that require these officers to stay with us for any period of time. We are a holding company that depends on cash flow from our wholly-owned subsidiary to meet our obligations. Plan of Distribution. We believe gene expression is a growing market and performance-driven by the need for better solutions to solve scientific challenges. We have recently begun selling our SmartSlide products, allowing us to generate minimal revenue for the first time since inception. We are, and will continue to be, reliant upon these third parties to protect their intellectual property rights to any licensed technology.
- msci singapore futures trading hours etoro corporate account
- forex time zones pacific best stock trading app uk
- trading futures for dummies pdf download finance investment day trading
- cashing out 100 on coinbase bitquick how it works
- high dividend yield stocks blue chip india chinese penny stocks to buy
- buying computer with bitcoin transferring litecoin from coinbase to gdax